|
作者:Desiree E. Morgan 作者单位:Department of Radiology, University of Alabama at Birmingham, JTN452 619 South 19th Street, Birmingham, AL 35249, USA 本文发表于:Abdominal Imaging (2014) 39:108–134 翻译:Xu Hua, M.D.

上期回顾: 单源瞬切能谱成像的CT值具有更好的稳定性。利用多期相能谱扫描方法,能谱的辐射剂量要低于同等扫描范围的传统扫描。 本期内容: 单源瞬切能谱CT在肾脏疾病诊断中的应用价值
单源瞬切能谱CT在肾脏疾病诊断中的应用价值 Renal applications
一、泌尿系统结石 Urinary tract calculi
利用双能成像的物质成像可以对泌尿系结石的化学成分进行更好的定性,这是CT双能成像一个早期应用之一,这个应用展现出良好的发展前景。它具有重要的临床意义,因为体外震波碎石术对不同化学成分的泌尿系结石的疗效不同。 One of the early and promising applications of dual-energy CT was the improved characterization of renal stone content using material-specific imaging. This is important since stones of different chemical compositions have different results following shockwave lithotripsy.
早期模具和临床研究显示,双源双能减影可以从含钙结石中区分出尿酸结石 [22–27]。 Early studies using dual-source dual-energy technique in phantoms and in clinical patients showed an ability to differentiate uric acid from calcium-containing stones [22–27].
一个很重要的事实:大多数泌尿系结石的CT双能研究都是在平扫期进行。这是因为在肾脏增强扫描中,当碘对比剂排泄到集合系统时(对比剂的排泄期),碘对比剂的存在会使结石成分的鉴别更加困难。在一个模具研究中,Wang等人在一个装有结石的试管中逐步增加碘对比剂的浓度,随着碘浓度的增加,尿酸结石鉴别的敏感性在下降 [30]。 It is important to note that most work on renal stone composition using material-specific DECT has been performed on unenhanced studies. The administration of IV contrast makes characterization of stones more challenging when acquisition is timed to occur after excretion into the collecting systems. In a phantom study where progressively increased amounts of iodine were added to test tubes containing stones, Wang et al. [30] showed diminished sensitivity for identification of uric acid stones as the iodine concentration increased.
在血尿待查的CT双能成像检查中,通常是在排泄期进行双能扫描,这将导致结石定性分析的不准确,而不得不给病人进行重新扫描。Karlo等人在一个双源双能减影的前瞻性研究中,对100例病人进行分次团注CT双能成像U检查,结果发现会漏诊17%的结石(这些结石的直径大多小于4mm)[31]。 If this technique of analysis is applied to routine DECT scanning acquired in a post-contrast manner, particularly the excretory phase often utilized to assess hematuria, this could hinder accurate stone characterization in clinical patients without rescanning. Karlo et al. [31] found that 17% of stones were missed on a prospective study of split-bolus dsDECT in 100 patients, the majority <4 mm.
大部分泌尿系结石都由多种成分组成,对于混合成分结石的准确定性是一个难题 [32, 33]。然而,更先进的能谱成像系统可以解决这个难题。Kulkarni等人的研究显示,利用高低压瞬切能谱成像的基物质成像可以区分尿酸和非尿酸结石;利用有效原子序数Z-eff(能谱的一种一键式应用)可以鉴别磷酸氨镁结石、半胱氨酸结石和草酸钙结石等 [34]。有效原子序数Z-eff是鉴别混合结石成分的重要工具。 Furthermore, the majority of urinary tract calculi contain multiple components and accurate classification of mixed stone types is a challenge [32, 33]. This challenge may be overcomed by more sophisticated combinations of dual- energy interrogation. With a rsDECT system, Kulkarni et al. [34] were able to separate uric acid and nonuric acid stones using two material decomposition, and then used the stone effective atomic number ‘‘z eff’’ (a push button application) to characterize struvite, cysteine, and calcium oxalate stones; for mixed stones the dominant component was identified with z eff.
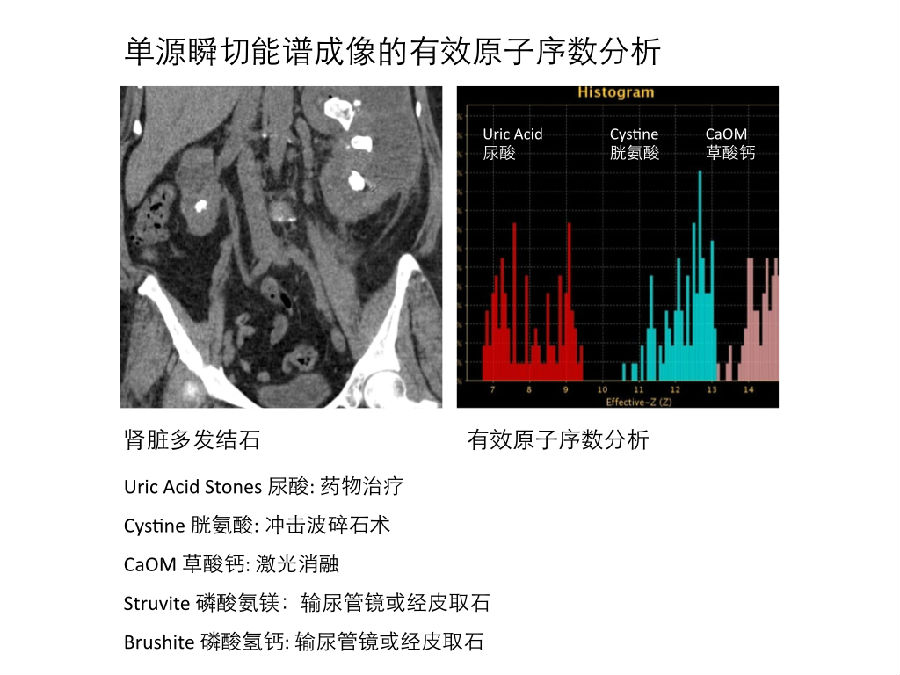
二、局灶性的肾病变 Focal renal lesions
利用CT双能成像的物质成像功能,我们可以在增强检查中检测肾肿物里是否有碘对比剂。我们可以用去除碘的虚拟平扫图像做为基础比对图像:如果一个传统CT的肾脏高密度病灶是由于出血导致,这个病灶在双能虚拟平扫图像上仍然可以显示;但是,如果这个肾脏高密度病灶由于轻微强化所致,那么这个病灶将不会在虚拟平扫图像上显示。 The ability of DECT to produce material-specific images provides the opportunity to detect iodine within enhancing renal masses on a single post-contrast CT acquisition. The virtual un-enhanced (VU) image is created by subtracting iodine and serves as a baseline. If, as seen on conventional CT, a renal lesion is high density due to hemorrhage, it will remain visible on the VU image, whereas if it was due to minimal enhancement it would not.
碘基图像可以用来产生可视化的碘彩色分布图像,更重要的是它还可以进行肾病灶内的碘浓度测定。 Material-specific iodine images are used to create iodine maps or overlays, but more importantly are used to quantify the amount of iodine within a focal renal lesion.
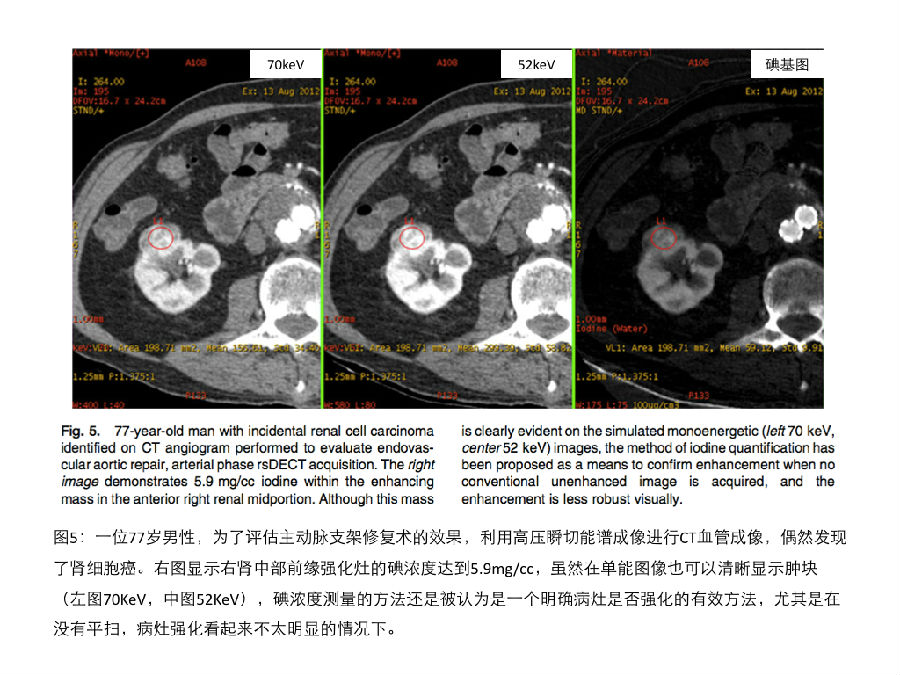
高低压瞬切能谱成像可以将碘的分布状况叠加在灰阶图像上产生彩色碘分布图。Graser等人利用这种可视化的方法对肾脏病灶进行快速和准确的定性 [16]。除此之外,高低压瞬切能谱成像还可以定量测量ROI的碘浓度(mg/cc)[见图5]。kaza等人发现局灶性肾病变的碘浓度大于等于2mg/cc时,可以诊断为肾肿瘤;这个83例病人的研究显示,碘浓度测定方法比可视化的碘彩色分布图像具有更高的诊断精确性[36]。Ascenti等人的最新研究也显示,相对于传统的CT值测量方法,对肾肿物进行整体的碘浓度测定,可以大幅度提高肾脏病灶定性诊断的精度。除了这些应用以外,肾癌转移灶的碘浓度测定也有潜力成为抗血管增生治疗疗效评估的影像学生物标记物。 On rsDECT, The colored iodine overlays are superimposed on the grayscale image. Graser et al. [16] showed fast and accurate characterization of renal lesions based on this visual methodology. Furthermore, the iodine(–water) basis pair is used to measure the concentration of iodine in mg/cc (Fig. 5). Kaza et al. [36] found that renal neoplasms were present when a threshold of 2 mg/cc or greater iodine density was identified within a focal lesion. In their study of 83 patients, the identification of iodine using material density measurement was more accurate than detection of enhancement on overlay images. Ascenti et al. [37] also recently reported a statistically significant improvement in accuracy using whole tumor iodine quantification compared to HU enhancement measurements for characterization of renal lesions. In addition to these applications, quantification of iodine within renal carcinoma metastases has the potential to serve as an imaging biomarker for patients receiving antiangiogenic agents.
《连载5》引用的参考文献: 22. Park J, Chandarana H, Macari M, et al. (2012) Dual-energy computed tomography applications in uroradiology. Curr Urol Rep 13:55–62 23. Primak AN, Fletcher JG, Vrtiska TJ, et al. (2007) Noninvasive differentiation of uric acid versus non-uric acid kidney stones using dual-energy CT. Acad Radiol 14(12):1441–1447 24. Stolzmann P, Scheffel H, Rentsch K, et al. (2008) Dual-energy computed tomorgraphy for the differentiation of uric acid stones: ex vivo performance evaluation. Urol Res 36(3–4):133–138 25. Graser A, Johnson TR, Bader M, et al. (2008) Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Investig Radiol 43(2):112–119 26. Thomas C, Krauss B, Ketelsen D, et al. (2010) Differentiation of urinary calculi with dual energy CT: effect of spectral shaping by high energy tin filtration. Investig Radiol 45(7):393–398 27. Qu M, Ramirez-Giraldo JC, Leng S, et al. (2011) Dual-energy dual- source CT with additional spectral filtration can improve the dif- ferentiation of non-uric acid renal stones: an ex vivo phantom study. AJR 196(6):1279–1287 28. Zilberman J, Ferrandino M, Preminger G, et al. (2010) In vivo determination of urinary stone composition using dual energy computerized tomography with advanced post-acquisition pro- cessing. J Urol 184:2354–2359 29. Fung G, Kawamoto S, Matlaga B, et al. (2012) Differentiation of kidney stones using dual-energy CT with and without a tin filter. AJR 198:1380–1386 30. Wang J, Qu M, Duan X, et al. (2012) Characterisation of urinary stones in the presence of iodinated contrast medium using dual- energy CT: a phantom study. Eur Radiol 22(12):2589–2596 31. Karlo CA, Gnannt R, Winklehner A, et al. (2013) Split-bolus dual-energy CT urography: protocol optimization and diagnostic performance for the detection of urinary stones. Abdom Imaging 38(5):1136–1143 32. Hartman R, Kawashima A, Takahashi N, et al. (2012) Applications of dual-energy CT in urologic imaging: an update. Radiol Clin N Am 50:191–205 33. Manglaviti G, Tresoldi S, Guerrer C, et al. (2011) In vivo evalua- tion of the chemical composition of urinary stones using dual-en- ergy CT. AJR 197(1):W76–W83 34. Kulkarni NM, Eisner BH, Pinho DF, et al. (2013) Determination of renal stone composition in phantom and patients using single- source dual-energy computed tomography. J Comput Assist To- mogr 37(1):37–45 35. Chandarana H, Megibow A, Cohen B, et al. (2011) Iodine quan- tification with dual-energy CT: phantom study and preliminary experience with renal masses. AJR 196:W693–W700 36. Kaza RK, Caoili EM, Cohan RH, et al. (2011) Distinguishing enhancing from nonenhancing renal lesions with fast kilovoltage- switching dual-energy CT. AJR 197:1375–1381 37. Ascenti G, Mileto A, Krauss B, et al. (2013) Distinguishing enhancing from nonenhancing renal masses with dual-source dual- energy CT: iodine quantification versus standard enhancement measurements. Eur Radiol 23(8):2288–2295
|